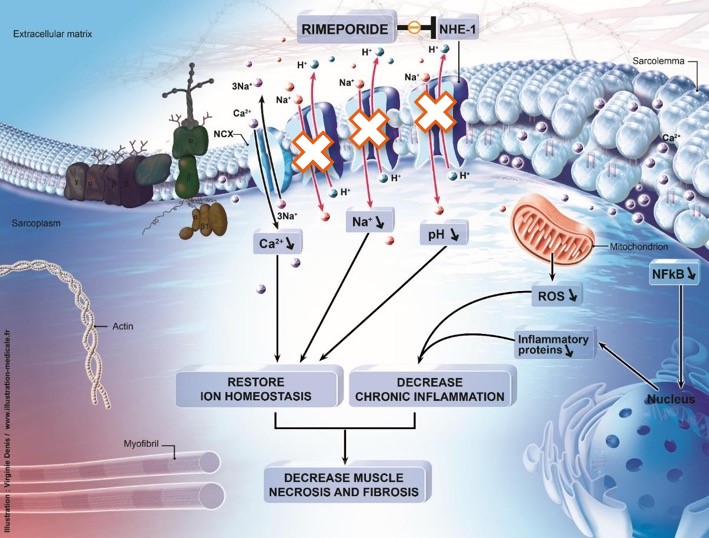
Publication of the results from the first European multicenter clinical trial of Rimeporide in young children with DMD

The trial evaluated the safety and tolerability profile, pharmacokinetics and biological activity of Rimeporide administered for 4 weeks to young boys with DMD.
Based on these positive results, 2 advisory meetings were held in May ( Eu and US Clinical Advisors and the Duchenne Data Foundation) in order to assist in the preparation of the phase II/III for what would be the 1st clinical study aimed at treating the cardiomyopathy affecting these children.
In addition to these discussions, EspeRare is also launching a call for partnerships to finance the pivotal clinical development of Rimeporide in cardiomyopathy.
To access the full text of the publication, click on the following link: https://doi.org/10.1016/j.phrs.2020.104999
About the journal Pharmacological Research:
Pharmacological Research publishes cutting-edge articles in biomedical sciences to cover a wide range of topics that advance the field of pharmacology. They offer a place where specialists from different disciplines can quickly exchange information on the health sciences that relate to modern pharmacological topics. The journal publishes articles on molecular, biochemical, translational and clinical research (including clinical trials)
