Advancing treatments with drug repositioning
Hand-in-hand with patients, we rescue and reposition drugs thus accelerating the development of accessible treatments for rare diseases
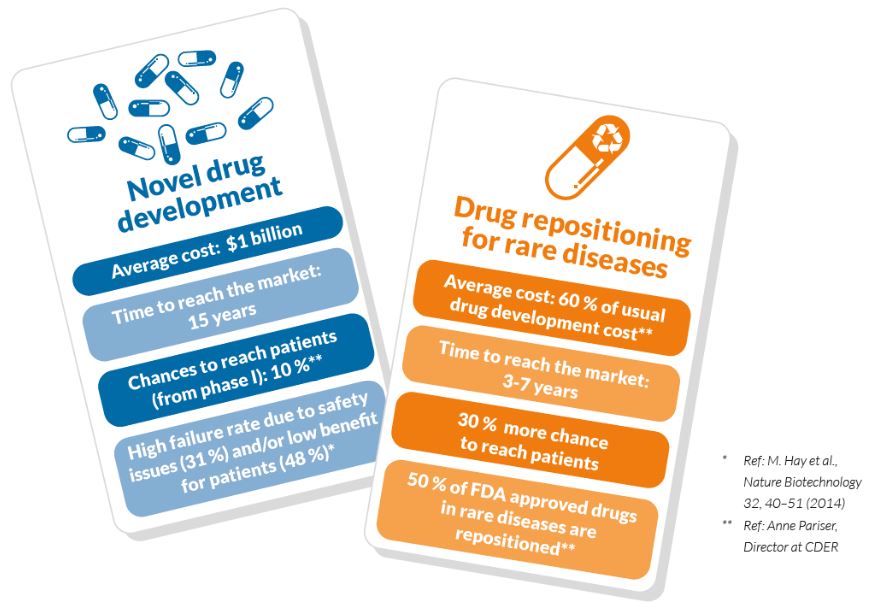
Finding new uses for existing drugs and rescuing discontinued treatments that have high potential in rare diseases is our focus in order to accelerate the development of life-changing treatments for these patients. Drug repositioning surpasses traditional drug development in terms of time and cost and increases the chance of success of bringing accessible treatments to rare disease patients.
Treatments we select have often been through extensive R&D testing such as toxicology, pharmacokinetic, safety and clinical assessments in healthy volunteers and in patients in other diseases. When repositioning these treatments in rare diseases, it allows us to build upon previous research and development efforts, thus accelerating and de-risking the path towards treating patients.
Selection criteria for drug repositioning
- Existing drugs already developed by pharma, biotech and academia are selected based on:
- > Toxicology and safety profiles
- > Understanding of their modes of action
- > Chemistry, manufacturing and control data packages
- > Clinical data packages
- > Business development paths towards full development and commercialisation
Once the repositioning opportunities are identified, the foundation applies its pharmaceutical expertise to conduct preclinical and early clinical assessments of these therapeutic opportunities. Thus developing its selected programmes until clinical proof of concept in patients is established. Upon positive results, EspeRare secures commercial partners for full development and commercialization, while securing a clear path for treatment accessibility to patients.