ER-004 Programme in X-Linked Hypohidrotic Ectodermal Dysplasia (XLHED)
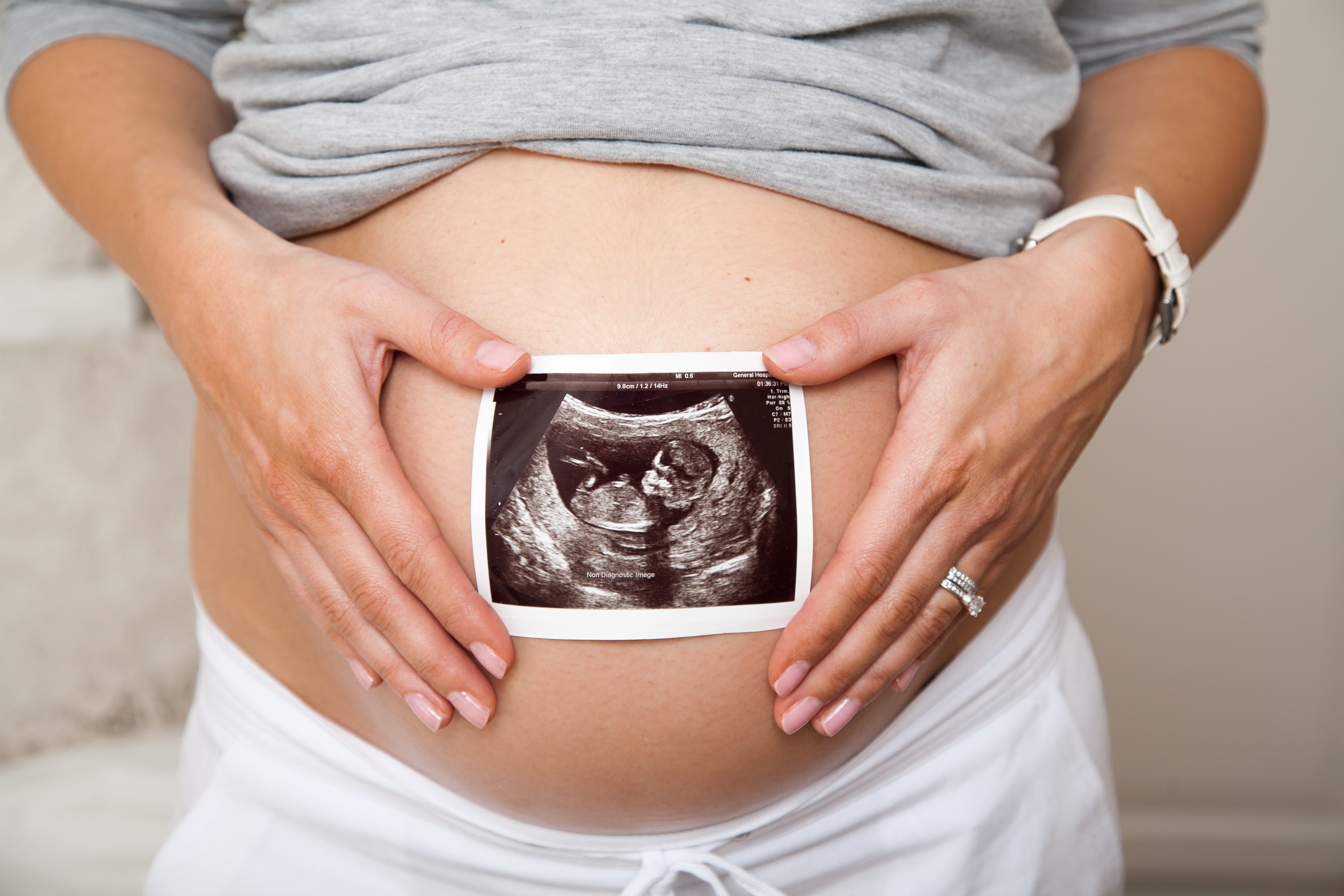
ER-004: A "single course" prenatal therapy to induce normal ectodermal development

ER-004 is a pioneering protein replacement therapy. ER-004 acts as a substitute for endogenous Ectodysplasin 1 protein, EDA1. EDA1 is a protein involved in signalling during early human development and missing in X-Linked Hypohidrotic Ectodermal Dysplasia (XLHED) patients. Administered before birth, ER-004 aims to trigger the normal development of key ectodermal structures such as glands, teeth and hair, structures affected in XLHED patients.
Prior to being developed by EspeRare, ER-004 was engineered in Lausanne, Switzerland, and further developed by Edimer Pharmaceuticals, a US-based biotech company.
ER-004 is the first and only treatment in development for XLHED
Administered at the right time during fetal-development, ER-004 has the potential to become a “single course” treatment, effectively switching off symptoms of the disease throughout patients’ lives. This approach has already demonstrated a significant potential in humans where it normalized sweat gland function in three patients treated in this fashion by Prof. Holm Schneider at the University Hospital Erlangen in Germany. Initial results were published in the New England Journal of Medicine [1] and in the British Journal of Clinical Pharmacology [2] as well as featured in Nature Medicine’s Research Highlights [3].
In 2020, EspeRare and the Pierre Fabre group signed an agreement to co-develop ER-004 towards commercialization as a prenatal treatment for XLHED. Together, EspeRare and Pierre Fabre have launched a pivotal clinical trial that gears to taking ER-004 to the market in the EU and the US. As part of their co-development agreement, both partners have committed to an Ethical Charter that governs their relationship and places the XLHED patient community at the heart of ER-004 development. (link to press release)
In the U.S., in addition to this FDA Breakthrough Therapy the program benefits from Fast Track and Orphan Drug Designation. In Europe, the program receives support from the EMA’s PRIME (Priority Medicines) and also the Orphan Drug Designation. With these multiple regulatory incentives, the program can aim at a streamlined worldwide development.
EDELIFE clinical study is recruiting
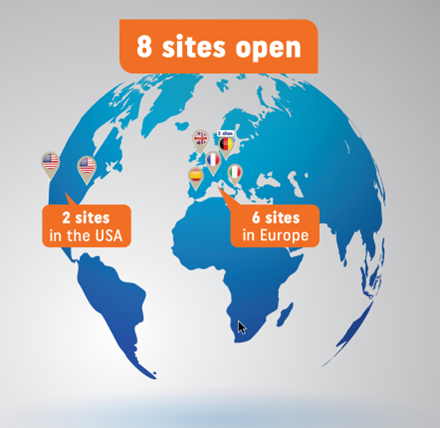
In 2021, EspeRare and the Pierre Fabre group launched the clinical study named EDELIFE that gears to taking ER-004 treatment to the market in the EU and the US in 2027. The aim of the EDELIFE clinical trial is to confirm the safety and efficacy of ER-004, given as a prenatal treatment to XLHED-affected boys.
The University Hospital of Erlangen in Germany was the first investigational site to open for recruitment of women pregnant with an XLHED-affected boy with Professor Holm Schneider as the principal investigator. Other investigational sites in Leipzig (Germany), Paris (France), Murcia (Spain), Milan (Italy), Cardiff (UK), Saint Louis, MO (USA) and Los Angeles, CA (USA), bringing the total number of sites for the EDELIFE clinical trial to eight, spanning six countries.
XLHED-affected families that wish to learn more about participating in the EDELIFE clinical trial should visit www.clinicaltrials.gov. The website is the source of information on study description, design, eligibility criteria, and it provides contact details of investigators and patients’ representatives.
Hand-in-hand with XLHED patients
EspeRare regards the ongoing engagement of the patient community as critical for the success of the ER-004 programme. The Foundation also acknowledges that the patient community has already greatly contributed to advance knowledge of this genetic condition (e.g., through efforts of collecting natural history data and providing feedback on characterization of the disease and unmet medical needs).
EspeRare and its partners are very grateful to families for sharing their experience with receiving an in-utero treatment for their children affected by XLHED.
“There were a lot of tears. But, I just knew it was a short term, small sacrifice for my son to possibly have a lifetime change” – says Emily, who embarked on a journey across the Atlantic to save her son from overheating and respiratory issues which are life-threatening symptoms, especially for young XLHED patients. To find out more on the Nelsons’ story, follow the link.
References
- [1] Prenatal Correction of X-Linked Hypohidrotic Ectodermal Dysplasia. Schneider H, Faschingbauer F, Schuepbach-Mallepell S, Körber I, Wohlfart S, Dick A, Wahlbuhl M, Kowalczyk-Quintas C, Vigolo M, Kirby N, Tannert C, Rompel O, Rascher W, Beckmann MW, Schneider P. N Engl J Med 2018; 378: 1604-1610
- [2] Safety and immunogenicity of Fc-EDA, a recombinant ectodysplasin A1 replacement protein, in human subjects. Körber I, Klein OD, Morhart P, Faschingbauer F, Grange DK, Clarke A, Bodemer C, Maitz S, Huttner K, Kirby N, Durand C, Schneider H. Br J Clin Pharmacol. 2020;86(10):2063-2069
- [3] In utero correction of a genetic disorder. Stower H. Nature Medicine 2018; 24: 702
ER-004 Expected patient benefits
- > Fewer febrile seizures
- > Reduced risk of brain damage
- > Reduced infant mortality
- > Fewer respiratory infections
- > Reduced ocular problems
- > Facilitation of implants and dental work
- > Better nutrition
About X-Linked Hypohidrotic Ectodermal Dysplasia (XLHED)
XLHED is a rare genetic disorder affecting ectodermal structures including sweat glands, respiratory glands, skin, hair and teeth. Clinical manifestations of XLHED are severe and can include life-threatening episodes of hyperthermia, heat intolerance, and an increased risk of serious respiratory tract infections. There are currently no approved therapies for the treatment of XLHED and the current standard of care is only palliative.
As boys have one single copy of this gene on their X-chromosome, they usually display the full spectrum of the syndrome while girls tend to present with a milder phenotype.
XLHED is debilitating and potentially life-threatening disease in the first years of life, when infants' lives are at risk due to hypothermia and/or severe respiratory infections.
Link to more "about XLHED".
ER-004 COMPASSIONATE USE/EXPANDED ACCESS POLICY in XLHED
Expanded access/Compassionate use refers to the use of an investigational therapy, outside a clinical trial, for patients with serious or life-threatening diseases or conditions who lack therapeutic alternatives. An investigational therapy is a medicine that has not yet been approved by goverment regulatory agencies.
EspeRare’s mission is to advance treatments for rare diseases and to promote treatment accessibility through a patient-centered approach.
Accordingly, a Patient Advisory Council dedicated to the XLHED program has been established and provides the opportunity to EspeRare and patient groups representatives to work in close collaboration. Clinical development plans are discussed to generate robust and necessary data for a market application and to contribute to the common goal of aiming at best patients' care and giving the greatest chance of success to this program. The ultimate shared objective is to maximize benefits and minimize risks for XLHED patients and their families.
Requests for compassionate use/expanded access will be considered on a case-by-case basis , in accordance with applicable regulations and in alignment with our mission and values and with the safety of the patient and childbearing mother as a priority. Of note, ER-004 is delivered to the patient before birth by injection into the amniotic fluid, an untried route of administration that requires careful consideration in each individual case.
Inquiries regarding expanded access may be sent to: info.er004@esperare.org
EspeRare will endeavor to acknowledge receipt of any expanded access questions or requests within 5 business days.