Addressing rare diseases
Rare diseases are a major healthcare burden
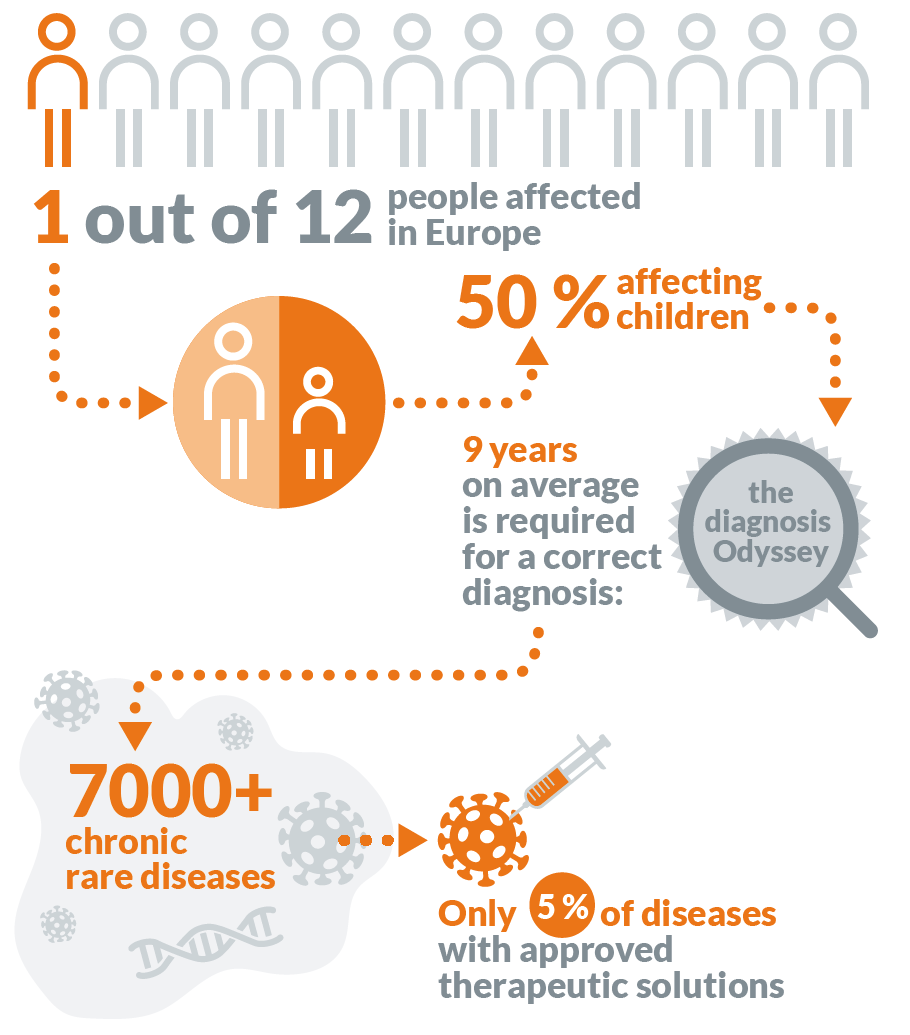
8 to 10% of the global population is affected by rare diseases; it includes diseases that impact fewer than 1 in 2,000 people. Unfortunately, only 5% of rare diseases (also called "orphan diseases") have approved treatments.
Most rare diseases are genetic, and thus are present throughout the person's entire life, even if symptoms do not immediately appear. Many rare diseases appear early in life, and about 30% of children with rare diseases will not live to celebrate their fifth birthday.
An opportunity to address a major gap in the medical landscape affecting children
Several therapeutic opportunities to treat rare disease patients exist but are too often left in “drawers” of pharmaceutical companies or universities. These opportunities are often never tested nor developed because biopharmaceutical companies are rarely willing to risk investing Research and Development (R&D) budget for this small market and lower financial return potential. Academia on the other hand often lack the know-how to conduct robust drug development especially in late phases of clinical development.
New hope for patients with life-threatening rare diseases
Developing new treatments is expensive, very lengthy and requires tight coordination between a large spectrum of research and development (R&D) activities and expertise. Therefore, EspeRare focuses on accelerating and increasing the chance of success of discovering and developing novel treatments for rare diseases by repositioning existing therapies.


